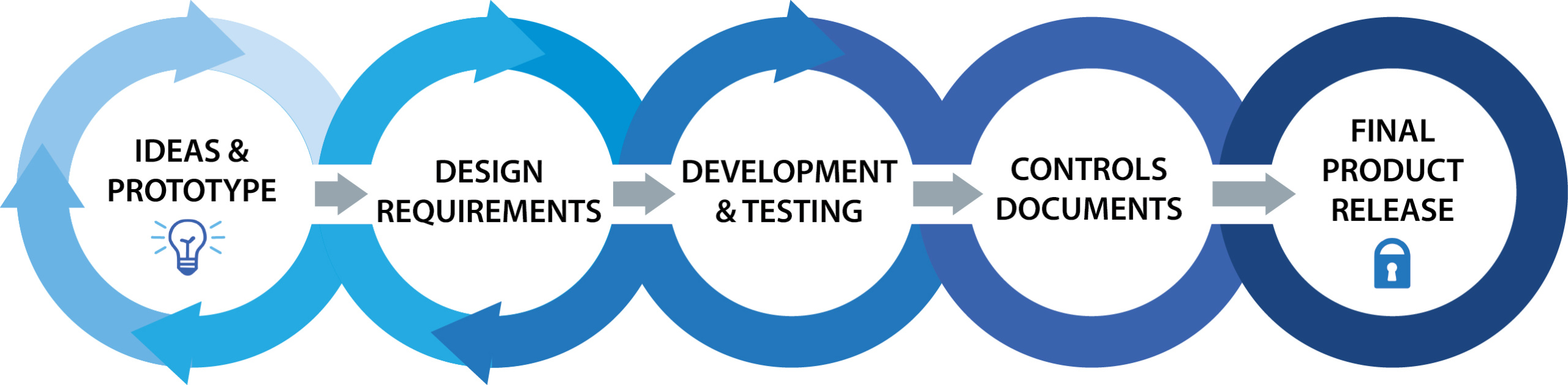
Custom Software
Tietronix provides FDA compliant design controls with efficient and transparent development solutions.
Mobile Apps
Tietronix has expertise is in managing mobile projects for multiple operating systems and various devices.
AI & ML
Tietronix can develop your AI engagement including massive parallel processing capability, trainable algorithms, and creating Agents for the top LLM(s)